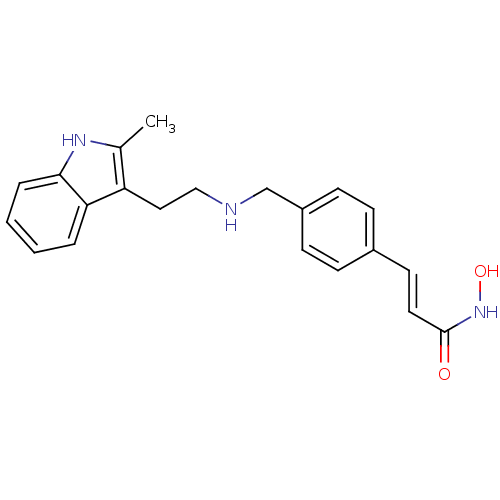
BDBM29589 Faridak::LBH-589::LBH-589B::Panobinostat::US10722597, Compound Panobinostat
SMILES Cc1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1
InChI Key InChIKey=FPOHNWQLNRZRFC-ZHACJKMWSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 29589
Found 9 hits for monomerid = 29589
Affinity DataKi: 5.10nMAssay Description:Inhibition of human recombinant HDAC10 using fluorogenic HDAC substrate after 45 mins by fluorimetrc methodMore data for this Ligand-Target Pair
Affinity DataKi: 31nMAssay Description:Competitive inhibition of HDAC10 using KI-104 as substrate by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of recombinant HDAC10 (unknown origin) using AMC labeled AC-peptide as substrate incubated for 1 hr by fluorescence analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:Inhibition of human recombinant HDAC10 expressed in baculovirus infected insect Sf9 cells using Ac-peptide-AMC as substrate assessed as release of AM...More data for this Ligand-Target Pair
Affinity DataIC50: 12.7nMAssay Description:The inhibitory activity of panobinostat dissolved in DMSO and that of the HP-β-CD panobinostat adduct (prepared as described in Example 1) were ...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibition of recombinant human His6/GST-tagged HDAC10 expressed in baculovirus infected High5 insect cells using Ac-Lys-Tyr-Lys(epsilon-acetyl)-AMC ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Inhibition of HDAC10 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:In vitro antagonistic activity against kinin-induced rabbit jugular vein contraction.More data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMAssay Description:Inhibition of human recombinant HDAC10 using Boc-Lys(triflouroacetyI)-AMC substrate incubated for 2 hrs by fluorescence based assayMore data for this Ligand-Target Pair